M. DMD medicine
Rare genetic diseases in children
evelopment of medicine for Duchenne Muscular Dystrophy (DMD)
-

Efficacy Test
-

Toxicity Test
-
Clinical Test
Source : https://auroshealthcare.wordpress.com/
Characteristics of the disease
- 80 to 85% of all patients with muscular dystrophy are DMD patients
- Ratio of nearly 1 in 3,500 newborn boys
- Symptoms such as posture deformation which typically begin at the age of 3-4, leading to gradual atrophy of muscles
→ Most die in their early 20s when respiratory muscles become too weak - Currently, a full recovery is not possible due to the absence of medicine, which increases the necessity of developing a new drug
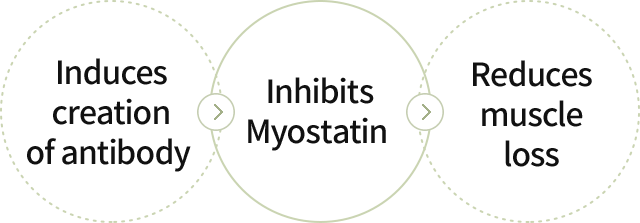
Selected Myostatin, an inhibitor of muscle creation, as the target antigen
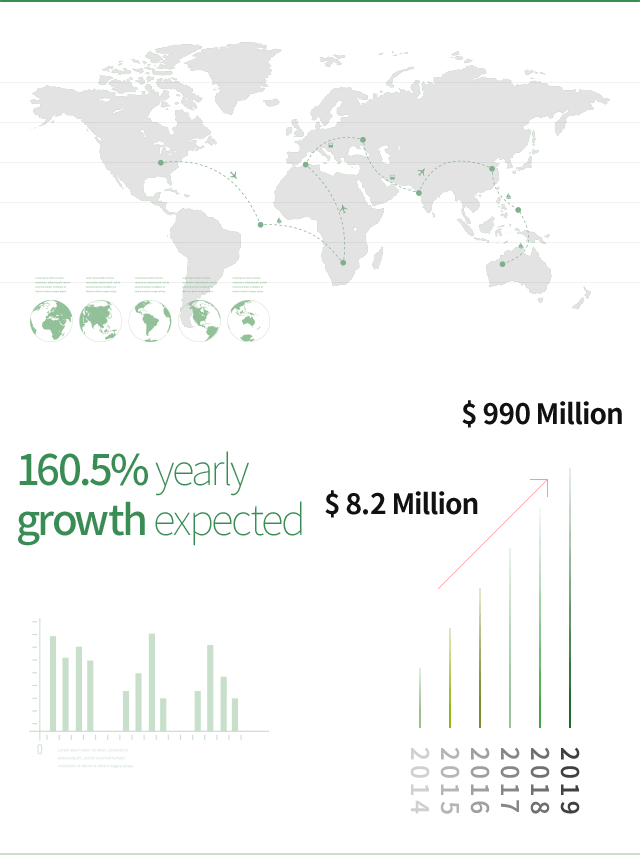
Global market value
Status
Approved with the Orphan Drug Designation (ODD) by the U.S. FDA in February 2018
→ Increased possibility for acquisition of PRV
Orphan Drug Designation
- Provision of research subsidies during the clinical test period
- Tax deductions for clinical test expenses
- Cost exemption for the review of new drug approval applications
- Permission of conditional sales after Clinical Phase 2
- Assignment of 7-year, exclusive marketing rights after permission is granted for marketing
- [SAMSUNG MEDICAL CENTER] Signed an agreement for joint R&D in January 2012
- Selected for and executed the assignment to develop advanced medical technology given by Ministry of Health and Welfare (2015 - 2018)
Strategy
- Complete Clinical Phase 2 · Acquire permission for marketing from U.S. FDA
- Secure additional technical partners → Establish a joint venture with a local partner or proceed with licensing out
- Acquire PRV
- A voucher received when developing an innovative new drug for rare diseases designated by the U.S. FDA : The period for approval of the development company's other medicines reduced to 6 months
→ Period of revenue may be extended, which leads to increases in sales - Possible to use or sell directly : The current sales value of PRV sits at over KRW 210 billion on average

- Status of PRV acquisition in relation to DMD disease
| Year | Company | Drug | Comments |
|---|---|---|---|
| 2017 |  |
Emflaza (deflazacort) |
FDA Press release |
| 2016 |  |
Exondys51 (eteplirsen) |
|
| Sarepta has sold PRV to Gilead for $125m |
 |
||
Source : http://priorityreviewvoucher.org